What We Lose Without the ACIP
The history and purpose of the ACIP, and what’s at stake as it’s dismantled.
I’m David Higgins, a practicing pediatrician and preventive medicine physician who cares for children, adolescents, and their families and studies how to improve how we communicate about and deliver preventive care and vaccines. This newsletter shares clear science, smart policy, and meaningful conversations, because the health of our communities depends on all three. If you haven’t already, hit the button below to stay in the loop with updates.
Hi Community,
Last week’s Advisory Committee on Immunization Practices (ACIP) marked a break from decades of scientific process, public transparency, and evidence-based decision-making.
I wrote about my core takeaways from the meeting in my newsletter last week. And here you will find pre-ACIP briefing and fact-checking documents I co-authored with colleagues. That fact-checking effort became necessary because the meeting was flooded with misinformation, some of it from people now shaping national vaccine policy.
Today, I’m circling back to part two of my series on the history of vaccine recommendations in the U.S. Understanding how the ACIP was built and why makes clear what’s at stake. Replacing it with a patchwork of recommendations and politicized committees isn’t a long-term solution.

Part 2: The Modern Era of Vaccine Recommendations
When the ACIP held its first meeting in 1964, its mission was to provide the U.S. Surgeon General with expert guidance on the effective use of vaccines and other preventive agents in public health, including recommendations on immunization schedules, target populations, and vaccination strategies. What followed over the next 60 years was a consequential and collaborative public health effort that helped prevent disease, save lives, and build one of the most trusted public health tools in the country: the national immunization schedule.
The ACIP Model: Collaborative by Design
From the beginning, ACIP recognized that no single committee could answer every scientific, clinical, and logistical question vaccines raise. That’s why it invited liaisons from professional organizations across the healthcare and public health landscape, with each bringing critical insight into how vaccines are used, delivered, and understood in the real world.
At its first meeting, ACIP included just three liaison organizations: the American Academy of Pediatrics (AAP), the American Medical Association, and Canada’s National Advisory Committee on Immunization. Three federal agencies joined as ex officio members: the Food and Drug Administration, the National Institutes of Health, and the Department of Defense. Over time, this list of liaison and ex officio members expanded to include family physicians, nurses, pharmacists, and public health leaders.
The involvement of multiple stakeholders allows the ACIP to translate evidence into action across various settings. Each member turns evidence into action in clinics, schools, supply chains, and communities.
A Pivotal Shift: The Birth of the Harmonized Schedule (1995)
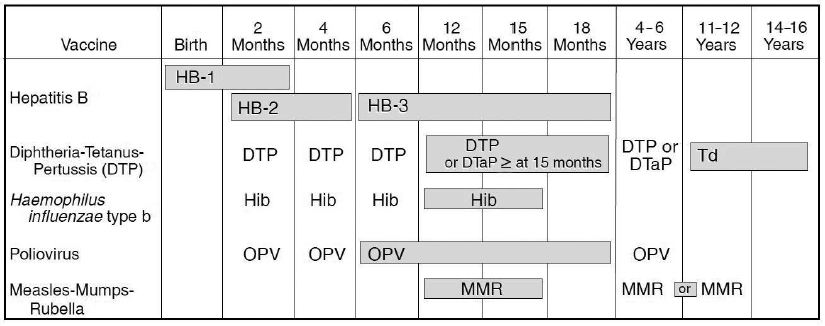
By the early 1990s, multiple groups were still issuing vaccine recommendations, including the ACIP, the AAP, and the AAFP. Each group’s recommendations had subtle wording, timing, or priority differences. For example, each group might agree that a vaccine was important but differ on the age at which it should be administered.
One of the clearest examples was the second dose of the MMR vaccine. The ACIP recommended giving it to children aged 4–6 to streamline delivery and boost early protection. The AAP recommended 11–12 years, aiming to create a two-dose cohort that would be better protected entering middle and high school, where outbreaks were likely to occur.
These differences created confusion not only for clinicians but also for schools, state health departments, and families.
In 1995, the ACIP, AAP, and AAFP collaborated to publish the first harmonized childhood immunization schedule, a unified guide endorsed by all three organizations. It outlined which vaccines children should receive and when, across all U.S. states and territories.
This harmonized schedule, updated annually, became a cornerstone of U.S. public health. It helped standardize care across all states and thousands of clinical settings. It supported vaccine mandates for school entry. It enabled insurers, vaccine manufacturers, and supply chains to plan efficiently. And most importantly, it reduced disparities by clarifying exactly what immunization every child in the U.S. was entitled to.
As you can see above in the original 1995 schedule, the MMR guidance wasn’t fully aligned initially, but in 2000, the AAP adjusted its recommendation to match ACIP’s.
Recommendations Grounded in Evidence
Over the decades, the ACIP has continually refined its decision-making processes. It adopted formal frameworks to bring structure, clarity, and transparency to recommendations.
Notably, the committee has shown it can revisit its own decisions when the science evolves. It has delayed or rejected recommendations when the data wasn’t strong enough. It has acted quickly during public health emergencies, like H1N1 in 2009 and COVID-19 in 2020, without abandoning scientific integrity.
Every ACIP meeting is open to the public. Votes are recorded. Evidence is shared. That transparency has been a cornerstone of how the ACIP operates.
And the ACIP is just one part of a broader vaccine ecosystem that includes regulatory review, safety monitoring, manufacturer oversight, and public health delivery systems, all reinforcing one another to build trust and effectiveness.
A Confusing Patchwork
So what happens when the ACIP is undermined?
We’re already seeing it.
Last week, I spoke with reporters about how Colorado prepared for this moment. This year, our state passed two new laws to protect vaccine access and uphold science-based policy.
Other states can follow Colorado’s lead, and many will have to. However, these efforts are temporary scaffolding, not a long-term structure.
As I wrote in The Washington Post:
“A patchwork of state protections is not ideal. Unified federal recommendations ensure parity in access and consistency across states—which is why the dismantling of the ACIP is so tragic. But as Ashish Jha said, ‘inaction is worse.’”
Vaccine confidence, delivery, and access are deeply tied to federal infrastructure, financing, supply chains, and unified travel policies. And of course, viruses don’t respect state lines. Fragmentation doesn’t just confuse providers; it invites inequity and delays.
Rebuilding Trust
The erosion of trust in federal health guidance isn’t new, but it’s accelerating. More and more Americans say they no longer trust the U.S. government to provide accurate vaccine information. This crisis of confidence didn’t start with the COVID-19 pandemic. It is the result of years of politicization, misinformation, and a steady erosion of transparency.
That’s why institutions like the ACIP matter. Not because they are perfect, but because they were designed to rise above political pressure and to make science-based decisions in the public interest. The ACIP wasn’t built to serve politics. It was built to serve the public. And when it works, it provides the clarity, consistency, and credibility that parents, providers, and policymakers depend on.
Bypassing that system is a breach of trust. When decisions are made behind closed doors, when evidence is ignored, and when conflicts of interest go unchecked, the foundation of public confidence collapses.
If we let that model fall, we lose a cornerstone that keeps kids healthy, vaccine-preventable disease outbreaks rare, and communities protected.
Thanks, as always, for being part of this community.
-David
Do you like this newsletter?
Then you should subscribe here for FREE to never miss an update and share this with others:
You can also follow me on LinkedIn, Instagram, Substack Notes, and Bluesky.
Community Immunity is a newsletter dedicated to vaccines, policy, and public health, offering clear science and meaningful conversations for health professionals, science communicators, policymakers, and anyone who wants to stay informed. This newsletter is free for everyone, and I want it to be a conversation, not just a broadcast. And if you find this valuable, please help spread the word!



ThankYou , Dr Higgins for writing about the importance of a strong, centralized and scientifically based national vaccine program and the history of the one we used to rely on. It’s important to speak out now more than ever.
David: Thank you for sharing the history of the ACIP. We had a similar experience with the CDC Lead Advisory Committee at the CDC. In 2002, Rep. Edward Markey and Senator Henry Waxman released a report titled “Turning Lead Into Gold: How the Bush Administration is Poisoning the Lead Advisory Committee at the CDC.” The report showed that the administration blocked CDC–nominated public health experts from joining the ACCLP and appointed members with industry ties, including connections to lead manufacturers and law firms associated with lead industries. It derailed policy for several years.